Table of Contents
Definition
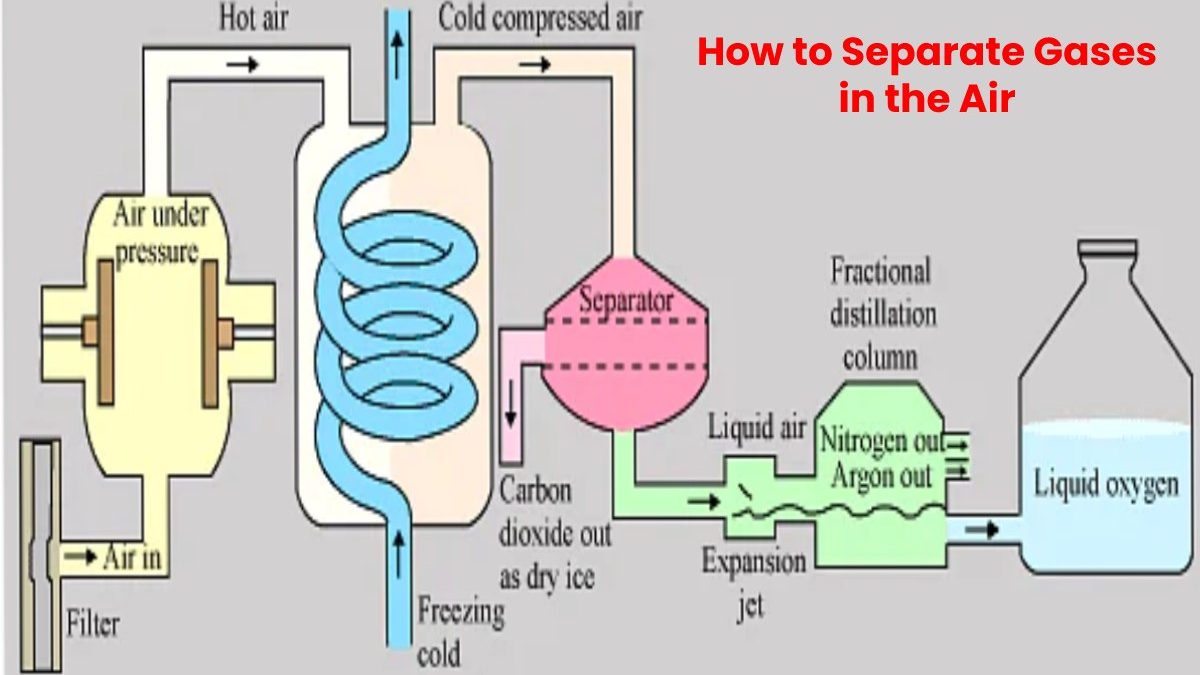
Gases in air can be separated through a process known as a fractional distillation of liquid air. It is a process that converts air into a liquid form and then allows it to be distributed in portions. Layered and separated from each other. It is a valuable technique since pure oxygen and nitrogen have various applications.
Fractional distillation is a process that can separate gases from each other if they can be convert into a liquid form. To perform this separation in air, it must be cool to a shallow temperature, so it liquefies. Once this is done, the air can pass to the bottom of a fractionating column. This column slowly raises the temperature until the oxygen and nitrogen in the air are separate from each other. From there, two tubes separate the gases separately.
What are Examples of Ideal Gases?
Some ideal gases are oxygen, nitrogen, carbon dioxide, and other gases in the Earth’s atmosphere. An ideal gas is a gas at low pressure and relatively high temperature in which the individual gas atoms or molecules can be assume to be far apart and not interact with each other.
The ideal gas law is an equation of state, describing a relationship between gas pressure (P), volume (V), number of moles present (n), and temperature (T), as follows: PV = now. R is the universal gas constant that varies with the units of the other factors in the equation. In words, the ideal gas law states that the molar density of a gas is proportional to pressure and temperature. The perfect gas law is assume valid at temperatures above room temperature (72 Fahrenheit) and pressures at or below atmospheric (1 atmosphere).
What are the Charges of the Noble Gases?
The noble gases have neutral charges. In addition, because these elements have complete outer electron shells, they are resistant to bonding with other factors. As a result, scientists long thought that these elements were completely inert.
The reactivity of elements is derive from the number of unfill electrons in the outermost valence shell. The noble gases comprise the periodic table column with filled outer surfaces. All noble gases have eight electrons in their outermost valence shells, except helium, which has only two. Because much of an element’s behaviour is govern by its reactivity, the noble gases behave similarly.
What is Air and It Separation?

Air can be separate into its components using distillation in special units. So-called air fractionating plants employ a thermal process known as cryogenic rectification to separate the individual components from one another to produce high-purity nitrogen, oxygen and argon in liquid and gaseous form.
Compression of Air
Ambient air is drawn in, filter and compress to approx.—six bars with a compressor.
Precooling of Air
To separate air into its components, it must first be liquefied at a shallow temperature. As a first step, the compressed air is precool with chilled water.
Purification of Air
Impurities such as water vapour and carbon dioxide are then remove from the air in a so-called molecular sieve.
Cooling of Air
Because the gases which make up air only liquefy at shallow temperatures, the purified air in the primary heat exchanger is cool to approx. -175°C. The cooling is achieve through internal heat exchange, in which the cold gas flows generate during the process cool the compressed air. Rapid pressure reduction then causes the compressed air to cool further, whereby it undergoes partial liquefaction. Now the air is ready for the separating column, where the actual separation takes place.
Separation of Air
The separation of air into pure oxygen and pure nitrogen in two columns, the medium-pressure and also the low-pressure columns. The difference in the boiling point of the constituents is exploit for the separation process. Oxygen becomes a liquid at -183°C and nitrogen at -196°C. The continuous evaporation and condensation brought about by the intense exchange of matter and heat between the rising steam and the descending liquid produce pure nitrogen at the top of the low-pressure column and also pure oxygen at the bottom. Argon is separate into additional columns and involves some extra steps.
Withdrawal and Storage
Gaseous oxygen and nitrogen are fed into pipelines for transport to users, e.g. steelworks. In liquid form, oxygen, nitrogen and argon are store in tanks and transported to customers by road tankers.
Separation Techniques: Obtaining Gases from Air
Fractional distillation is a separation method where the difference in boiling points of components is use to separate the liquid mixture into fractions through distillation. The process begins with the liquefaction of air. Let’s try to understand the process with the help of an illustration of the separation of nitrogen from the perspective.
To obtain nitrogen gas from the air and also we need to remove the rest of the constituents of the air. So, before we start, the air is filter to remove the dust particles and then liquefied.
Conclusion: Gases in Air
Air can be separate into its components using distillation in special unitsand also Hence, So it called air fractionating plants employ a thermal process known as cryogenic rectification to separate the individual components from one another to produce high-purity nitrogen, oxygen and argon in liquid and gaseous form.